10 Feb 02/10/2022
Sterile Gloves vs. Non-Sterile Gloves
Disposable gloves are used by working professionals both in and out of the medical field every day to provide a liquid barrier to one's hands, reduce the spread of microorganisms, and protect workers from harmful substances. However, some individuals must wear a more advanced disposable glove, often called sterile gloves, free from germs. Like non-sterile disposable industrial- and medical-grade gloves, sterile gloves are made from nitrile, latex, or vinyl. Since the same materials create both sterile and non-sterile gloves, it brings up two crucial questions:
- Do you know the difference between sterile and non-sterile gloves?
- Are you sure you know when to wear the correct style?
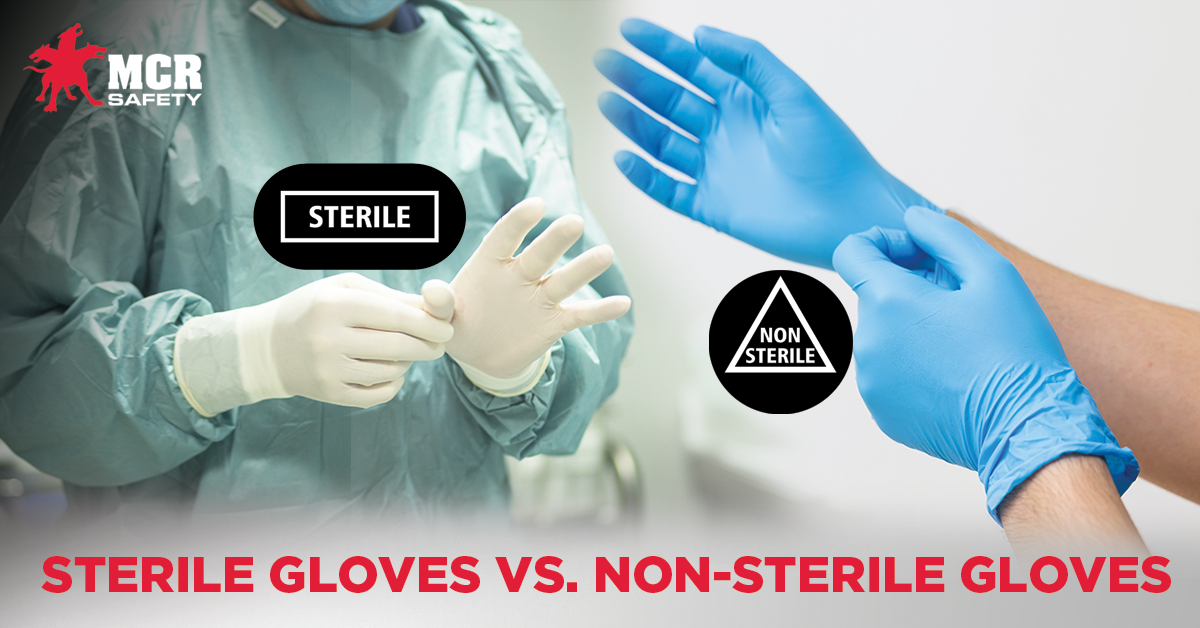
Sterilization is defined as removing all living organisms, such as bacterial or fungal spores, from a surface. Numerous medical environments require sterile gloves, ranging from chemotherapy procedures to surgical operations. While MCR Safety only stocks non-sterile gloves in our product lineup, we understand users must know the differences between these two glove types to select and wear the correct product at all times. Below, we'll cover what distinguishes each type of glove from the other, their applications, and how to use them properly. We'll also address some common questions about sterile and non-sterile gloves.
What are sterile gloves?

Sterile gloves are an essential form of personal protective equipment (PPE) that prevents the spread of pathogenic organisms and protects people from harmful illnesses. These gloves are manufactured under special conditions and with superior processes, ensuring the gloves meet the highest standards possible to minimize the risk of spreading bacteria in specific medical applications.
Sterile gloves are irradiated with gamma waves to remove dangerous germs, making them safe for medical use in hospitals, doctor's offices, and other healthcare settings. A Certificate of Irradiation (COI) declaration must accompany these gloves, validating the gamma irradiation has occurred. In addition to gamma radiation, there are two other sterilization methods used for medical devices such as gloves:
- Autoclaving – high-pressure steam and heat
- Ethylene oxide – gas
Sterile gloves are rigorously examined across every production sequence before arriving at the final end-user. They must pass stringent quality assessments, often backed up with a Certificate of Analysis (COA) report confirming specified lot numbers. These gloves have a lower Acceptable Quality Level (AQL) than non-sterile gloves, often scoring a 1.0 or less. AQL indicates a glove's performance level for withstanding liquids and the probability of pinhole defects. The lower the number, the greater the glove's likelihood of holding up against liquids. After sterilization, gloves must be individually packaged and placed in more robust secondary packaging material than non-sterile gloves. Lastly, the gloves must be transported, stored, and handled under special conditions, ensuring the sterilization of each glove.
In the United States, sterile gloves will often meet two standards from regulatory agencies: the Association for the Advancement of Medical Instrumentation (AAMI) and the American Society for Testing and Materials (ASTM). These agencies ensure products meet safety standards that protect people from harm. The United States Food and Drug Administration (FDA) provides the final say in whether a sterile glove is truly sterile by assessing its sterility assurance level (SAL).
When should sterile gloves be worn?

The risks for transmitting disease increase during high-risk procedures involving bodily tissue. So, the primary intent of sterile gloves is for use during surgical procedures, where the potential spread of infection is high. According to the CDC, sterile surgical gloves should be worn in healthcare settings when doctors, surgeons, and other healthcare workers perform invasive patient procedures. Examples include any diagnostic or surgical procedure involving broken skin, body orifices, body fluids, or internal organs. Here are additional examples of situations identified by the World Health Organization when surgical gloves are worn:
- Administering chemotherapeutic agents
- Any surgical procedure
- Invasive radiological techniques
- Preparing total parental nutrition
- Vaginal delivery
Non Sterile Gloves Meaning

What are non-sterile gloves? Simply put, they are made from the same materials as sterile gloves: latex, nitrile, or vinyl. However, non-sterile gloves have not been treated with gamma waves or any other type of high-level decontamination method. They're appropriate for use in some healthcare settings that don't involve exposure to biological risks and are primarily used to protect medical personnel from secretions, fluids, and waste.
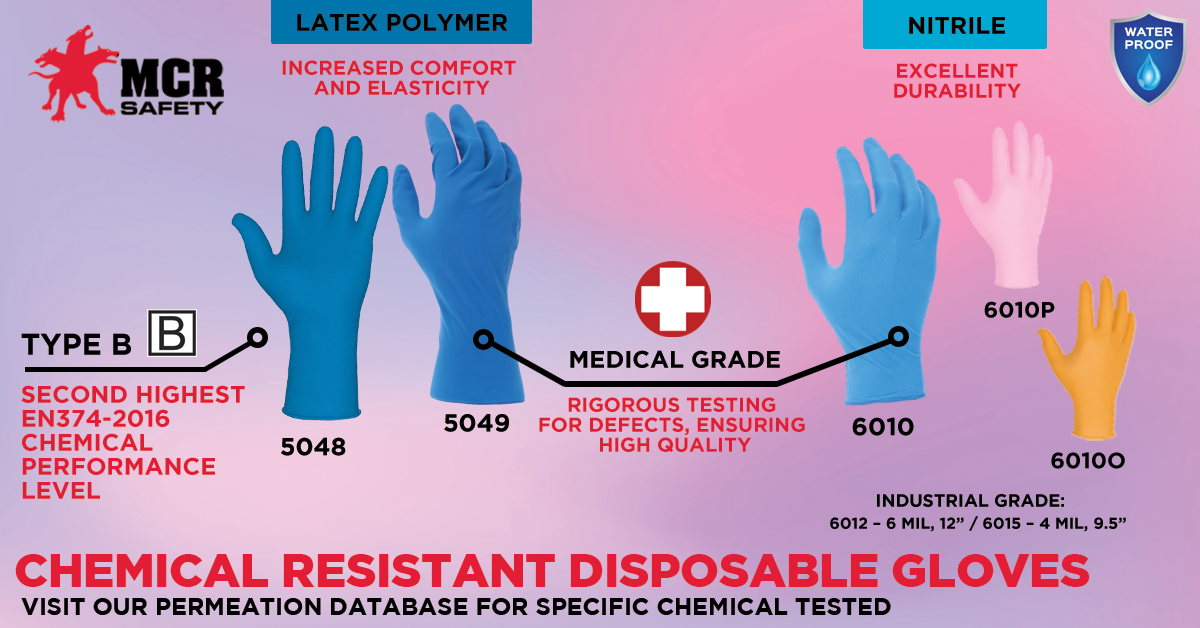
These gloves are offered in medical-grade exam gloves or industrial-grade disposable gloves. The difference between these two types involves the AQL mentioned above, as medical-grade gloves must achieve a minimum AQL of 1.5. Gloves that achieve a score of 1.5 and above are considered industrial-grade. Additional information on medical-grade performance can be found on our EN-374 educational page.
Non-Sterile Glove Applications

When there is minimal risk of contact with infectious bodily fluids or contaminated materials, non-sterile gloves can be a viable alternative to more expensive sterile gloves. Non-sterile gloves are worn in thousands of healthcare settings that involve low patient contact, like at the intake desks of doctor's offices. They are also used during routine examinations, which means they are frequently used in nursing homes, extended care facilities, rehabilitation centers, dialysis centers, and testing or diagnostic centers. They can be worn when performing low-contact activities with minimal risk of pathogenic exposure, like helping bedridden patients with their hygiene and cleaning non-sharp medical instruments and devices.
Medical Grade
Here are some situations identified by the World Health Organization when medical-grade, non-sterile exam gloves are often worn:
- Cleaning up spills
- Contact with blood and mucous membranes
- Handling and cleaning medical instruments
- IV insertion and removal
- Presence of highly infectious organisms
Science experiments are another setting where medical-grade options are worn, primarily due to the chemical resistance achieved.
Industrial Grade
When there is no potential of being exposed to blood or bodily fluids, non-medical grade disposable gloves are worn. Here are some examples identified by the World Health Organization:
- Administering injections
- Giving oral medications
- Taking blood pressure, temperature, and pulse
- Removing bed linens
- Transporting patients
Non-sterile gloves for food contact are where a good chunk of MCR safety non sterile gloves are worn.
MCR Safety's Non-Sterile Gloves

MCR Safety provides companies with both medical-grade and industrial-grade non-sterile disposable gloves. All of our options are highlighted on our disposable glove online glove catalog. Below are some of our top-selling styles in both categories:
Medical-grade means fewer pinholes, which also means better chemical resistance.
We now stock biodegradable disposable gloves, too!
12" length styles available.
Common Questions About Sterile and Non-Sterile Gloves

Are nitrile gloves sterile?
- Nitrile gloves may or may not be sterile, depending on the type. Never assume that nitrile gloves are sterile unless expressly stated on the box or container the gloves came in.
What does the term "non-sterile" gloves mean?
- Non-sterile gloves have not been treated with a procedure like gamma irradiation that removes germs and sterilizes the gloves.
What should nurses do when using sterile gloves?
- The most critical movement a nurse should make when donning sterile gloves is to keep their gloved hands raised above the elbows. This prevents bacteria or bacteria-laden moisture from moving toward the hands and contaminating them.
How should sterile gloves be put on?
- The application of sterile gloves should always follow the same safe and effective routine to prevent biological contamination. This procedure is commonly known as the "sterile glove routine." Apply the first glove and roll down its cuff, then use the gloved hand to grasp the other glove by its cuff and apply it to the ungloved hand. Reverse this cuffing procedure to remove the gloves without contamination.
Your Source for Non-Sterile Gloves

MCR Safety offers a wide variety of non-sterile gloves. Even though we don't offer sterile gloves, we believe in educating our customers and ensuring they know the differences and distinguishing factors of all PPE worn. It's a part of our commitment to protecting people. We want to ensure you're wearing the correct product for the application you perform.
Click the below image to leave us comments, questions, or any concerns.
For over 45 years, MCR Safety has proven to be a world leader in gloves, glasses, and garments. Whether it's hiking your favorite trail or working in winter weather, we are there providing solutions to cold weather hazards. It's all part of our commitment to protect people.
No matter your industry, we have the personal protective equipment you need.

Learn more about MCR Safety by checking out our most recent video. For more information, browse our website, request a catalog, find a distributor, or give us a call at 800-955-6887.
About the Author
Latest Articles